The human immune system is an incredible success story of evolution. It defends against a constant barrage of external threats – bacteria, viruses, and other pathogens – and, as I’ve recently learned, protects against an intrinsic threat: cancerous cells. In their review “Natural and Adaptive Immunity to Cancer“, Vesely and colleagues draw from recent mouse models of cancer and human clinical data to describe how cells, effector molecules, and pathways of the immune system act to suppress and control tumor cells. It’s not all good news, however. Apparently, certain immune system pathways (e.g. inflammation) instead serve to promote tumor growth.
The Immune System Strikes: Senescence and Apoptosis
Cells already have an array of intrinsic defense mechanisms that halt the transformation process. Numerous cellular proteins detect DNA damage and induce senescence, a permanent change of state characterized by morphological and gene expression changes. The activation of oncogenes, too, can trigger senescence. In fact, the hijacking of Ras signaling to escape senescence and proliferate is a key requirement for cell transformation. Alternatively, cells that sense injury or loss of mitochondrial integrity may undergo programmed cell death (apoptosis). This process may also be initiated externally by the ligation of tumor necrosis factor (TNF) family ligands to their corresponding receptors: TNF, TNF-related apoptosis-inducing ligand (TRAIL), and Fas ligand (FasL). There are still other, non-apoptotic paths to cell death (necrosis, autophagy, mitotic catastrophe) that are gaining attention as barriers to transformation.
How the Immune System Prevents Cancer
The immune system has three key responsibilities when it comes to preventing cancer:
- Suppression of viral infections, which when unchecked can induce certain kinds of tumors
- Timely elimination of pathogens, to reduce the extent and duration of inflammation, which often promotes tumorigenesis
- Immunosurveillance, in which transformed cells are identified and destroyed before they can establish malignancy.
The idea that the immune system might recognize and destroy tumor cells was conceived 50-100 years ago. This concept of “immunosurveillance” remained controversial, and saw little progress until the 1990’s. Does this story sound familiar? It’s much like the story of cancer and the metabolism, which also saw a long period of general ignorance before its “rediscovery” in the 1990’s. Mice get the credit for rekindling interest in the immune system’s tumor suppressor potential. Specifically, mice that were immunocompromised after loss of interferon (IFN) signaling or T-cell function. Such animals were significantly more susceptible to sarcomas after exposure to methylcholanthrene (MCA), implicating a role for the immune system in preventing these tumors in healthy mice.
Over the last 10 years, work from many labs (including the authors’) has demonstrated how the immune system works to prevent outgrowth of many types of primary and transplanted tumors. The RAG2-knockout mouse, which is deficient in T-cells, B-cells, and natural killer (NK) cells, develops more spontaneous cancer lesions and is also more susceptible to MCA-induced sarcoma. Interestingly, a significant portion (40%) of tumors that develop in RAG2-knockout mice are rejected when transplanted to immunocompetent (wild-type) mice, demonstrating that normal immune system function successfully suppresses these cells. Sarcomas induced in wild-type mice (with MCA), however, grow unrestricted when transplanted to other mice. These observations suggest a dual role for the immune system: in wild-type mice, it protects against tumor development, but also edits the immunogenicity of developing tumors, allowing them to grow unimpeded when transplanted to healthy mice.
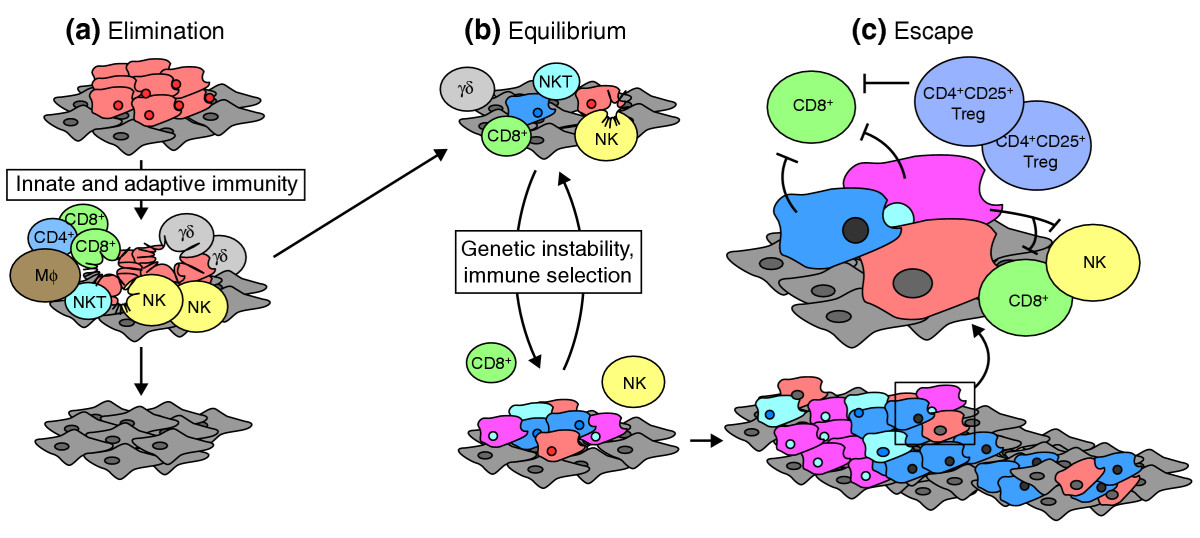
The Three E’s: Elimination, Equilibrium, and Escape
The authors have come to view immunoediting as a dynamic process with three distinct phases:
Credit: Strausberg, Genome Biol. (2005) 6:211
- Elimination, when innate and adaptive immune cells work together to identify and destroy tumor cells before a malignancy can form.
- Equilibrium, a phase when the immune system contains tumor outgrowth but does not eliminate transformed cells entirely.
- Escape, in which tumor cells grow unrestricted by the immune system, and develop into clinically apparent disease.
Both elimination and equilibrium might be considered satisfactory clinical endpoints for a patient, because tumor cells are either destroyed entirely or held in check to prevent outgrowth of disease.
The transition from equilibrium to escape is facilitated, at least in part, by the micro-evolution of the tumor cells during equilibrium. The selective pressure of immune recognition and destruction selects for tumor cells that are less immunogenic. Also aiding tumor escape is the breakdown of the immune system, either naturally (as a person ages) or as a direct result of immunosuppression (often induced by the tumor).
The Mouse Evidence: Knockout and Induced Tumors
Humans and mice have similar immune systems, with a largely overlapping repertoire of immune cells and effector molecules. The development of mouse strains deficient for specific genes, and the induction of tumors by carcinogens MCA (sarcoma) and DMBA/TPA (papilloma) have demonstrated that NK cells and cytotoxic lymphocytes (CTLs) suppress tumor initiation and growth in vivo. Interferon signaling also plays a key role in immunosurveillance, as demonstrated by the increased tumor susceptibility in mice lacking perforin, IFN-γ, IFNGR1, TRAIL, IL-12, TNF-α, and DNAM-1.
Numerous cytokine molecules and receptors have also been implicated in controlling induced tumors. Mice deficient in IL-12, for example, develop increased numbers of papillomas than wild-type mice. Interestingly, mice lacking IL-23 or IL-17A are resistant to tumor development, suggesting a tumor-promoting role for these cytokines. Interestingly, DMBA/TPA exposure in mice lacking the TRAIL receptor did not affect the number of induced tumors, but did increase the rate of metastasis to lymph nodes (compared to wild-type mice), indicating a role for TRAIL-R in suppressing metastasis.
Aging Studies and Spontaneous Tumor Development
The incidence of spontaneous tumors in normal mice is very low, possibly because they have long telomeres. Many strains of immunodeficient strains fail to develop tumors even after two years of observation. Aging studies in knockout mice, however, have elucidated the roles of certain genes, effector molecules, and immune cells in the defense against spontaneous tumors. This is an elegant type of experiment that requires some patience; one simply removes specific components of the murine immune system and monitors them for spontaneous tumor development. One striking discovery highlighted in this review was the incidence of immunogenic B-cell lymphomas, which increases from 0-6% in wild-type mice to 40-60% in mice lacking perforin, a cytolytic protein used by NK cells and T-lymphocytes. Penetrance of lymphomas in these mice is even higher when they also lack MHC class I accessory molecules (B2M) or IFN-γ. These observations support the importance of “cytotoxic” immune cells in protecting against spontaneous tumors.
Aging experiments have also been performed in mice lacking specific immune cell types. RAG-2 knockout mice, for example, develop significantly more ephithelial tumors (35% gastrointestinal, 15% lung), even when raised on broad-spectrum antibiotics in a pathogen-free facility. RAG-2 knockouts that also lack STAT1, a key player in interferon I/II signaling, develop an earlier and broader spectrum of malignancy, including colon and mammary adenocarcinomas.
Loss of Equilibrium
The equilibrium phase, in which the immune system holds tumors in check but fails to eliminate them entirely, is an interesting phenomenon. Here we observe a dynamic balance between a powerful immune system response and a genetically heterogeneous population of tumor cells that can persist for a number of years. It has become clear that adaptive immunity, and not innate immunity, takes the lead in controlling tumor outgrowth. This has been demonstrated by experiments in which healthy mice are subjected to low levels of carcinogen exposure (which tends to induce few tumors) and later depleted for CD4+/CD8+ T-cells and/or IFN signaling. As many as 50% of apparently tumor-free mice develop sarcomas at the injection site upon this depletion, suggesting that micro-tumors were present but held in check by adaptive immunity. Granted, the tumors that arise after immunodepletion tend to be highly immunogenic; when transplanted to healthy mice, 40% are rejected by the competent immune response. In contrast, sarcomas obtained from mice that were not immunodepleted tend to grow progressively when transplanted.
The Human Evidence: Immunodeficency and Immunosuppression
Although we have fewer experimental liberties with human subjects, clinical and epidemiological data have proven useful. Human patients with specific perforin mutations, for example, not only develop familial hemophagocytic lymphohistocytosis as adults, but have recently been shown to also develop leukemia and lymphoma. Surveillance of human patients with AIDS has shown an increased frequency of several malignancies due to the immunodeficiency. Most often, these tumors are induced by pathogens, such as Epstein-Barr virus (lymphoma), herpesviruses (Kaposi’s sarcoma), and human papilloma virus (cervical cancer) that fail to be eliminated by the deficient immune system.
Intentional immunosuppression in the recipients of organ transplants can also increase the risk of cancer. Patients receiving kidney transplants, for example, exhibit a three-fold increase in overall malignancy. Most of these, too, are virus-associated tumors, though there’s also an increased risk for colon, lung, pancreas, and other non-infectious cancers. Renal transplant patients are a dramatic example; these individuals have a 200-fold (yes, two hundred) risk for non-melanoma skin cancers, highlighting the importance of immunosurveillance in tumors induced by exposure to UV radiation. Further, the duration of pharmacology-induced imunosuppression and incidence of cancer are positively correlated; that is, the longer the immune system is suppressed, the more likely a tumor will form. Taken together, these observations support the importance of immunosurveillance in preventing human cancers.
Further evidence of the immunity-cancer relationship, particularly the equilibrium phase, is offered by the occasional organ recipients who develop cancer that originated from the organ donor. I’m horrified to hear that this can happen, but it does. Often, the donors had died of other causes and bore no signs of clinically-detectable disease, suggesting that their immune systems had held cancerous cells in check. The combination of a naive immune system, and immunosuppressive therapies required for successful engraftment, allows these tumors to grow without restriction in the unfortunate recipient.
Miracles Happen: Spontaneous Tumor Regression
Perhaps the most compelling evidence for the anti-cancer role of the immune system is the spontaneous regression of melanoma tumors accompanied by T-cell clonal expansion. This phenomenon suggests the ability of CD4+ and CD8+ T-cells to identify tumor-specific antigens and destroy cancerous cells. As many as 100 tumor-associated antigens (TAAs) generate an antibody response in patient serum, though only 8 have been observed in multiple studies. This suggests that TAAs, much like somatic mutations, are largely unique to individual tumors. T-cell responses vary from antigen to antigen; for example, responses to MAGE family antigens are rare, whereas responses to melanocyte differentiation antigen (MART/Melan-A) are seen in >50% of healthy individuals.
More studies are needed here to catalogue TAAs and quantify their antigenicity across patient populations. Here, too, is where high-throughput sequencing of tumor genomes might offer useful information as well. Knowledge of the full set of protein-coding mutations in a tumor might shed light on its immunogenic potential, or vice-versa, thereby leading to better informed prognoses and treatment decisions.
Tumor-Infiltrating Lymphocytes and Disease Prognosis
Even without complete tumor regression, the presence and quality of tumor-infiltrating lymphocytes (TILs) – NK cells, T-cells, and NKT cells – has a favorable prognosis for numerous tumor types. This correlation was first observed in melanoma, where patients with high CTL infiltration of their tumors survived longer. A “landmark” study in ovarian cancer found that 38% patients with high TIL numbers survived longer than 5 years, compared to 4.5% of patients with low TIL numbers. Studies in colon and lung cancers have found that the type and density of TILs was more powerful prognostic indicator than the clinical stage of the tumor.
There is, of course, a downside to TILs: when they’re macrophages or regulatory T cells. High numbers of these can have a poorer prognosis, possibly due to their immuno-suppressive functions.
Inflammation and Tumor Development
Chronic inflammation can contribute to cancer by inducing genotoxic stress, cell proliferation, angiogenesis, and even enhancing tissue invasion. Even so, the tumor-promotion activities of inflammation and tumor-suppressing actions of the immune system are not mutually exclusive. In the authors’ mouse model of MCA sarcoma, for example, tumor development requires several inflammation molecules (MyD88, IL-10, IL1B,and IL-23), but these factors induce the host-protective immune response (IFN and T-cells) that destroy the tumors. In other primary carcinogen models, MyD88 and IL1B promote tumor development, but also facilitate the recognition of dying tumor cells that leads to anti-tumor immunity.
Another important role of inflammation is the transition from equilibrium to escape, when inflammatory and regulatory immune cells are recruited to the tumor, and then subverted to dampen anti-tumor immunity, allowing cancer progression. Indeed, the authors suggest that pro-inflammatory transcription factors NF-KB and STAT3 may be valuable therapeutic targets, whose inhibition may facilitate the transition from tumor-promoting inflammation to tumor-suppressing immunity.
References
Vesely MD, Kershaw MH, Schreiber RD, & Smyth MJ (2010). Natural Innate and Adaptive Immunity to Cancer. Annual review of immunology PMID: 21219185