Massively parallel sequencing will be applied to hundreds or thousands of tumor genomes this year. Catalogues of somatic alterations in human cancers (e.g. COSMIC) will grow, perhaps as exponentially as dbSNP did in the past decade. Perhaps more importantly, we will begin to see cases where whole-genome or whole-exome sequencing of a patient’s tumor guides his or her treatment. Bridging the gaps between mutation discovery, biological interpretation, and clinical action, however, will be a substantial challenge. Hence the theme of this month’s posts, cancer biology and pathology.
Just over a decade ago, Douglas Hanahan and Robert A. Weinberg published the landmark article “The Hallmarks of Cancer” in the journal Cell. At the time, nearly a quarter-century of rapid advances had revealed a wealth of knowledge about this deadly disease. Although more than 100 subtypes of cancer had been described, Hanahan and Weinberg described six principal cellular traits shared by virtually all forms of human cancers. Collectively, these essential alterations in cell physiology dictate tumor development and growth.
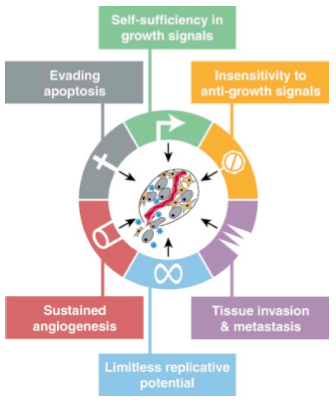
Credit: Hanahan and Weinberg, Cell (2000) 100:57-70
Each of these six acquired capabilities – evasion of apoptosis, self-sufficiency in growth signals, insensitivity to growth inhibition signals, limitless replicative potential, sustained angiogenesis, and tissue invasion/metastasis – represents the successful circumvention of inherent anticancer defense mechanisms of cells and tissues.
Genomic Instability and Driver Mutations
Most of these acquired capabilities arise from somatic alterations – mutations, structural events, and epigenetic changes – which presents something of a dilemma. Thanks to a swath of fastidious DNA monitoring and repair enzymes, mutation is a rare event, and altering the critical genes to successfully acquire each capability is inefficient. For a single cell to achieve all of them in the span of a human lifetime is, well, statistically improbable. The authors suggest a seventh principle, not a hallmark of cancer but a universally enabling characteristic, to explain the means by which these six biological endpoints are reached: genomic instability.
Mutations that cause genomic instability are likely critical, early events in tumorigenesis. Studies have shown that mutations in DNA repair genes (e.g. ATM, RAD51, CHEK1) and, more recently, components of DNA methylation pathways (DNMT3A/DNMT3B) are recurrently mutated in human cancers, suggesting an important functional role in disease development.
Hallmark 1: Self-sufficiency in Growth Signals
Autonomous growth signaling was the first hallmark to be defined by cancer researchers, due in part to the large number of oncogenes that modulate it. Three common molecular strategies are used by tumors to provide self-sufficient growth stimulation:
- Modulation of extracellular growth signals, for example, the production of PDGF and TGF-alpha by glioblastomas and sarcomas, respectively.
- Alteration of the trans-cellular signal transducers (surface receptors), e.g. the up-regulation of EGFR in stomach/brain/breast tumors and HER-2 in mammary tumors.
- Deregulation of the intracellular signaling pathways linked to transmembrane receptors, such as the Ras/Raf/mitogen activated protein kinase (MAPK) cascade.
The authors suspected that growth signaling pathways suffer deregulation in all human tumors. Ten years later, we know that this is largely true. Large-scale sequencing efforts have revealed that mutations in Ras-family genes (KRAS, NRAS, HRAS, etc.) and MAP kinase genes are frequent events in human cancers. In breast cancer, for example, PI3 kinase genes are among the most highly mutated, suffering alterations in as many as 40% of tumors.
Hallmark 2: Insensitivity to Antigrowth Signals
In normal tissue, both soluble factors and matrix-embedded inhibitors cooperate to maintain homeostasis by blocking cell growth. Much like growth stimulation, these signals are transduced to cells via transmembrane receptors and then into complex intracellular circuits. At the molecular level, most or all anti-proliferative signals are funneled through retinoblastoma (Rb) related proteins. When hypophosphorylated, Rb sequesters and alters the functions of E2F transcription factors, which normally serve to activate a number of genes required for transition from G0 to S-phase.
The best documented modulator of Rb signaling is TGFB, a soluble signaling molecule that suppresses cell growth. TGFB prevents the phosphorylation that activates Rb, thereby blocking the advance through G1. Tumor cells disrupt TGFB signaling by a number of mechanisms, including downregulation or alteration of its cellular receptor, or mutation of the key transducer of TGFB signaling, Smad4. One way or another, the anti-growth circuit converging on Rb is disrupted in a vast majority of human malignancies, virtually “defining the concept” of tumor suppressor loss in cancer.
Hallmark 3: Evasion of Apoptosis
I won’t dwell much on this topic, since much of it was covered in my post on Cancer Versus the Immune System. Simply put, evasion of apoptosis is a hallmark of many and perhaps all human cancers.
Hallmark 4: Limitless Replication Potential
Most types of mammalian cells carry intrinsic programs that limit their replication. Once cells reach a certain number of divisions, they stop growing, or senesce. In culture, human fibroblasts can be forced to keep dividing (by knocking out p53 and pRb tumor suppressors) beyond this point. These cells eventually enter a crisis state characterized by karyotypic disarray and massive cell death. A small fraction of cells, however, continue to grow and divide without limit, a trait known as immortalization.
The limit for most normal cell types is 60-70 divisions, after which they enter senescence. Obviously tumor cells surpass this limit, managing to grow and progress even while undergoing massive apoptosis. One key rate-limiting mechanism in cell division is the length of chromosome telomeres, which decreases by 50 to 100 base pairs with each consecutive cell division. At some point, telomere loss introduces massive genetic instability, and crisis ensues.
Many, if not all tumor cells address this issue by up-regulating telomerase, the enzyme that extends telomeres. This maintenance operation is a key component for enabling limitless replication potential in tumor cells.
Hallmark 5: Sustained Angiogenesis
Here’s something I didn’t know: virtually all cells must remain within 100 um of a capillary blood vessel to get the oxygen and nutrients they need to survive. You’d think that because of this limitation, rapidly proliferating cells must have an intrinsic ability to induce angiogenesis (blood vessel growth). It turns out, not so much. The ability to stimulate angiogenesis is not inherent in normal cells or developing neoplasias, and represents an acquired capability that successful tumors must achieve.
Like the other biological processes discussed in this review, angiogenesis is encouraged or prevented by a complex network of signaling molecules. Soluble factors, cell surface receptors, integrins, and cell adhesion molecules all play a role in the counter-balancing of blood vessel growth. Vascular endothelial growth factor (VEGF) and fibroblast growth factors (FGF1/FGF2), for example, are molecules that stimulate angiogenesis initiation. Thrombospondin-1 is an important inhibitor of this process.
Tumor cells encourage blood vessel invasion/growth by up-regulating inducers and suppressing inhibitors, often at the level of transcription. Loss of TP53, for example, causes thrombospondin-1 levels to fall, thereby reducing the latter’s inhibitory potential. Similarly, Ras activation and loss of the VHL tumor suppressor induce an up-regulation of the gene encoding VEGF. More recently, we’ve come to realize that the transmembrane receptors for angiogenesis-stimulating molecules (VEGFR, FGFR) are commonly mutated in human cancers.
Hallmark 6: Tissue Invasion and Metastasis
Eventually, tumor cells venture out from the primary lesion to colonize and grow in other, often distant parts of the body. Ultimately, it is these metastases that account for 90% of cancer deaths. Several families of proteins involved in tethering cells to their surrounding tissue are altered during this process. Perhaps the best-known of these are cell-cell adhesion molecules (CAMs) and integrins, which mediate cell-cell interactions and cell-matrix interactions, respectively. One example of the communication between cell and environment is offered by E-cadherin, which is expressed on the surface of epithelial cells. Bridging of E-cadherin receptors between adjacent cells triggers anti-growth signals within the cell (Lef/Tcf transcription factor activation) via cytoplasmic B-catenin. A number of epithelial cancers block this pathway, either by mutational inactivation of E-cadherin or B-catenin genes, transcriptional repression, or proteolysis of the extracellular E-cadherin domain.
Integrins have dozens of subtypes with distinct substrate preferences. Successful colonization by tumor cells at a distant site requires adaptation, which is often achieved through shifts in the spectrum of integrin alpha- and beta-subunits displayed by migrating cells. Support for this idea can be seen even in cell culture, where forcing expression of different integrin subunits can induce invasive and metastatic behavior. This aspect of tumor progression will be especially challenging to characterize, because there are large numbers of integrin genes and many, many unique heterodimeric receptors that can be generated by differential subunit expression.
Summary
It was clear even a decade ago that the innate defense mechanisms of cells to prevent transformation and metastasis are diverse and complex, and the processes by which tumor cells subvert those defenses are equally so. Nevertheless, Hanahan and Weinberg postulated that 10-20 years after the time of writing this review, diagnosis of virtually all somatic lesions within a tumor would be a routine procedure, as would comprehensive gene expression analysis. With such knowledge in hand, it would be possible to definitively test whether all tumor types behave according to a set of common rules like the ones outlined above. We aren’t quite able to provide those answers just yet, but given the rapid advances of next-gen sequencing, that day is soon coming.
References
Hanahan, Douglas, & Weinberg, Robert (2000). The Hallmarks of Cancer Cell, 100 (1), 57-70 DOI: 10.1016/S0092-8674(00)81683-9
