The 2009 Nobel Prize in Physiology/Medicine was shared by three researchers “for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase.”
- Elizabeth H. Blackburn, now at the University of California, San Francisco.
- Carol W. Greider, now at Johns Hopkins University
- Jack W. Szostak, now at Harvard Medical School / Massachusetts General Hospital.
In response to the award, Scientific American made available a very nice article (Telomeres, Telomerase, and Cancer) written by Greider and Blackburn in 2006. In it, the authors chronicled the work of their predecessors as well as their own travails that eventually yielded the Nobel-prize-winning discoveries.
Discovery of the Telomere
In the 1930s, two geneticists working independently and with different model organisms – Barbara McClintock at the University of Missouri-Columbia (my alma mater) and Hermann J. Muller at the University of Edinburgh – found that chromosomes had “caps” on their ends that provided stability. Muller coined the term “telomere” and McClintock found that chromosomes without telomeres stick to one another or underwent structural changes that were bad news for the cell housing them.
Telomeres and the End-Replication Problem
In the 1970s, the makeup of telomeres (6-nucleotide repeats, or telomeric subunits) was determined. It differed across species, and the number of telomeric subunits varied from cell to cell, even within a single organism. Telomere size fluctuated over time as well. In 1972, our friend James D. Watson observed that DNA polymerases could not copy linear chromosomes all the way to the tip, which meant that some sequence at each end of a chromosome goes uncopied during DNA replication. Blackburn and others reasoned that there must be a way for cells to compensate for this constant shortening of telomeres.
It was in 1984 that Blackburn and Greider, working together at UC Berkeley, set out to find the agent that maintained telomere length in cells. Experiments with Tetrahymena cells showed that something in the cell extracts – an enzyme – had the ability to lengthen telomeres by adding subunits. The mystery agent, telomerase, is made up largely of protein. However, it contains one additional key element – a single-stranded RNA template from which new telomeric subunits are made.
A Factor in Human Aging
In complex organisms such as humans, not all cells express telomerase. Indeed, while telomerase is found in cells of the germline – no doubt to preserve telomeres in constantly-dividing germ cells – most somatic cells don’t have it. This means that as cells divide, their telomeres are shrinking. Somatic cells from a human newborn will divide 80 to 90 times when placed in culture, yet the same cells from a 70-year-old human will divide only 20-30 times. When cells that normally undergo division stop doing so, they “senesce” – change in appearance and eventually die.
Intuitively, this provides a very neat model for human aging, whose common hallmarks (e.g. wrinkles, weakened immunity, loss of hair) seem tied to the loss of dividing cells. Could it be that telomerase, then, might offer immortality?
Telomerase and Cancer
It appears so, though not in the long-sought Fountain of Youth manner that one might hope. Instead, telomerase plays a key role in the immortality of many forms of cancer. In 1996, Harley and Shay (the latter at UT Dallas) found that telomerase was expressed in 90 of 101 tumor samples representing 12 different cancer types, but none of 50 control samples of somatic cells. Greider, Harley and others, in working with virus-induced models of cancer, found that transformed cells divide continuously – shortening their telomeres as they go – and at some critical point, most of them die. Some few, however, begin expressing telomerase when their telomeres are dangerously short, and survive. It seems that telomerase activation is not the initiating event in tumor development; instead, it confers immortality to cells that are already cancerous.
As Blackburn and Greider point out in their article, this offers a very intriguing (if hypothetical) model for the role of telomerase in cancer. Normally, in the course of human development telomerase activity is suppressed in the cells of somatic tissue. These cells divide continuously until reaching some critical shortening of telomeres, at which they stop. If, however, somatic mutations occur that confer the ability to ignore that “stop” signal, the cells may transform. Loss of the telomeres would result in dangerously unstable chromosomes, which might explain the crazy cytogenetics and structural changes often observed in cancers. Many such cells will die, but some fraction may acquire changes that allow them to become immortal, such as the activation of telomerase.
It could be, however, that telomerase plays only an ancillary role in the development of most human cancers. Only further research, perhaps with tumors that have many structural/cytogenetic abnormalities, will define the part that telomeres and telomerase play in tumorigenesis.

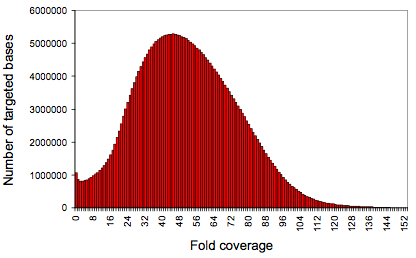
 g of input DNA), Ng et al selectively targeted CCDS regions totaling 26.6 Mb of sequence (~0.83% of the human genome). Capture specificity was similar to that of other published methods (35-55% of reads mapping to targets), but the
g of input DNA), Ng et al selectively targeted CCDS regions totaling 26.6 Mb of sequence (~0.83% of the human genome). Capture specificity was similar to that of other published methods (35-55% of reads mapping to targets), but the