Inflammation was first linked to cancer in the 1863 by Rudolf Virchow, who observed that inflammatory cells were present in tumor biopsy specimens, and tumors often developed at sites of chronic inflammation. Investigations into this connection waned over the next century, and only recently have seen a resurgence of interest. A slew of new evidence has begun to unravel the molecular pathways by which these two conditions are associated, and led to a general acceptance of Virchow’s proposition. Three review articles in Nature, Carcinogenesis, and Oncology summarize what we know, and what we need to find out, about cancer-related inflammation.
Evidence for the Inflammation-Cancer Connection
Several lines of evidence support a link between chronic inflammation and cancer.
- Chronic inflammation increases the risk of several cancers. The best-established example comes from patients with inflammatory bowel disease; some 43% develop colorectal cancer after 25-35 years.
- The tumor microenvironment contains mediators of inflammation, both cellular (TAMs, T-cells) and molecular (cytokines/chemokines).
- The incidence and spread of cancer are reduced by anti-inflammatory drugs (NSAIDs and selective COX-2 inhibitors).
- Many oncogenes (e.g. MYC, RAS) and tumor suppressors (e.g. TP53) act upstream of inflammation signaling pathways, notably NF-κβ and STAT.
- In animal models, inflammation promotes the proliferation and survival of malignant cells, angiogenesis, immunoevasion, metastasis, and reduced sensitivity to chemotherapy.
I should emphasize here that inflammation refers to chronic, but not acute, inflammation. The latter seems to have no association with cancer in humans.
Two Pathways: Extrinsic and Intrinsic
At a high level, there are two pathways leading to cancer-related inflammation.
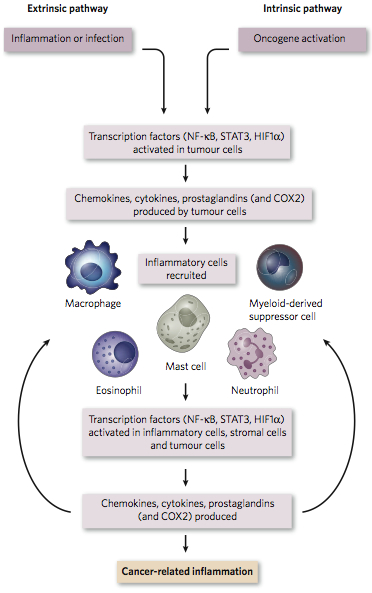
Credit: Mantovani et al, Nature 45|24, 2008
In the extrinsic pathway, inflammatory conditions due to environmental factors (e.g. infection and carcinogen exposure) facilitate tumor development. Take lung cancer, for example. Exposure to tobacco smoke (which contains 10^15 to 10^18 free radicals per gram) often causes chronic obstructive pulmonary disease (COPD) and/or low-grade emphysema, both of which are chronic inflammatory conditions that increase risk of lung cancer. In mice, H. influenza induces COPD-like inflammation that promotes lung cancer driven by the KRAS oncogene.
In the intrinsic pathway, somatic alterations in malignant cells activate signaling pathways that lead to the construction of an inflammatory micro-environment. In papillary thyroid carcinoma, for example, genomic rearrangements often activate the RET oncogene, which drives transcription of several inflammatory genes:
- Interleukin-1β, one of the main pro-inflammatory cytokines
- COX-2, which is involved in prostaglandin synthesis and up-regulated in many tumors
- CCL2 and CCL20, chemokines that attract monocytes and dendritic cells
- Chemokines IL-8 and CXCR4, which promote angiogenesis
Key Players: NF-KB and STAT3
Among the numerous molecules involved in cancer-related inflammation, transcription factors nuclear factor-kappaB (NF-κβ) and signal transducer activator of transcription 3 (STAT3) play pivotal roles. NF-κβ is a key orchestrator of innate immunity, activated by the toll-like receptor pathway (sensing microbes and tissue damage), as well as inflammatory cytokines TNF-α and IL-1β. Once activated, NF-κβ induces the expression of inflammatory cytokines, adhesion molecules, angiogenic factors, nitric oxide synthase, and COX-2. It also induces anti-apoptotic genes (i.e. Bcl-2), making it an attractive target for malignant cells. Indeed, NF-κβ expression is disregulated in many human cancers.
The maintenance of NF-κβ expression in tumors requires STAT3. Not surprisingly, STAT3 is often constitutively activated in tumor cells. In colon cancer, it plays a central role in cell proliferation and survival by regulating c-Myc, cyclin D, Bcl-2, and other genes. In lung cancer, mutations in key oncogene EGFR lead to activating phosphorylation of STAT3. Also, mouse models of colorectal cancer have shown that knockout of STAT3 (either by genetics or pharmacologic inhibition) reduces tumor development.
Tumor Recruitment of Immune Cells
Some of the chemokines present in the tumor micro-environment serve to recruit certain immune cells, notably tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). TAMs are a major source of cytokine production in the tumor micro-environment, and also provide a number of growth-promoting factors. TAMs come in two kinds: M1 macrophages, which respond to interferon or microbe exposure, and express high levels of anti-microbial/anti-tumor cytokines (e.g. IL-1, IL-6, IL-12, and TNF); and M2 macrophages, which promote angiogenesis and tissue remodeling. Cytokines and cells in the tumor microenvironment influence whether TAMs become M1 or M2. Both NF-κβ and MDSCs promote polarization towards M2 macrophage, thereby stimulating angiogenesis and tissue remodeling favorable for tumor growth. MDSCs also induce the maturation of CD4+ T cells (T-regs), which suppress the immune response in protective fashion.
The Role of Genetic Instability
Inflammatory cells and molecules can destabilize the genomes of cancer cells through a variety of mechanisms. They induce the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which can damage DNA by inducing double-stranded breaks. While cells have repair mechanisms for DSBs, they can be error prone. Further, nitric oxide and certain pro-inflammatory cytokines suppress TP53, which protects tumor cells from apoptosis triggered by DNA damage. IL-6, an inflammatory cytokine, promotes hypermethylation of tumor genomes by increasing DNA methyltransferase activity. Chronic inflammation has also been linked to disregulation of mitotic checkpoint proteins, which normally halt cell division until DNA damage is repaired.
These mechanisms act in concert to destabilize cancer genomes, allowing somatic mutations and rearrangements to occur at an increased rate. This leads to a genetically heterogeneous population of tumor cells, which are under selection for their ability to proliferate, evade host defenses, and invade other tissues. Thus, cancer-related inflammation promotes tumor growth by accelerating the somatic evolution by which malignant cells arise.
References
David W. Kamp, Emily Shacter, Sigmund A. Weitzman (2011). Chronic Inflammation and Cancer: The Role of the Mitochondria. ONCOLOGY, 25 (5).
Colotta F, Allavena P, Sica A, Garlanda C, & Mantovani A (2009). Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis, 30 (7), 1073-81 PMID: 19468060
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation Nature, 454 (7203), 436-444 DOI: 10.1038/nature07205